Thermodynamics! An awesome field, with many important equations. We gathered the top 10 of these formulas, just for you!
1. Thermal Conductivity – heat flow rate
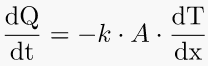
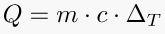
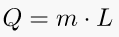
Latent heat is the energy released or absorbed by a body or a thermodynamic system during a constant-temperature process. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. A specific latent heat (L) expresses the amount of energy in the form of heat (Q) required to completely effect a phase change of a unit of mass (m), usually 1kg, of a substance as an intensive property. Intensive properties are material characteristics and are not dependent on the size or extent of the sample.
4. Thermal efficiency of a heat engine
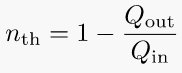
5. Thermal energy of an ideal gas
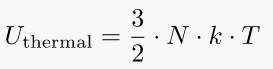
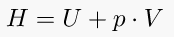
7. Gay-Lussac's Law (Pressure-temperature law)
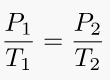
This law holds true because temperature is a measure of the average kinetic energy of a substance; as the kinetic energy of a gas increases, its particles collide with the container walls more rapidly, thereby exerting increased pressure.
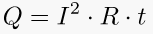
9. Boltzmann's Entropy Formula
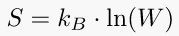
In short, the Boltzmann formula shows the relationship between entropy and the number of ways the atoms or molecules of a thermodynamic system can be arranged.
10. Van der Waals equation of state
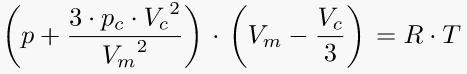
You can try to search whatever you want just by clicking on the “Browse Formulas” button on the top left of your screen.
If you need any help, you will find some in our fxSolver video.
Also remember to follow or interact with us in our social media pages. You will find links below.
