Osmosis (and Not Ozzmosis dear metalhead) is the spontaneous net movement of solvent molecules through a semi-permeable membrane into a region of higher solute concentration, in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a semipermeable membrane (permeable to the solvent, but not the solute separating two solutions of different concentrations). In other words, when a semipermeable membrane (animal bladders, skins of fruits and vegetables) separates a solution from a solvent, then only solvent molecules are able to pass through the membrane.
The osmotic pressure is defined to be the minimum pressure required to maintain an equilibrium, with no net movement of solvent. It is also defined as the measure of the tendency of a solution to take in water by osmosis. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity.
The osmotic pressure of an ideal solution, with low concentration can be approximate calculated by the molarity and the temperature of the solution.
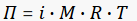
As always, you can try using fxSolver, with the Osmotic pressure (Morse equation) formula in our database.
Please, remember to follow our social media pages, by hitting the buttons below and watch the fxSolver video as well!
